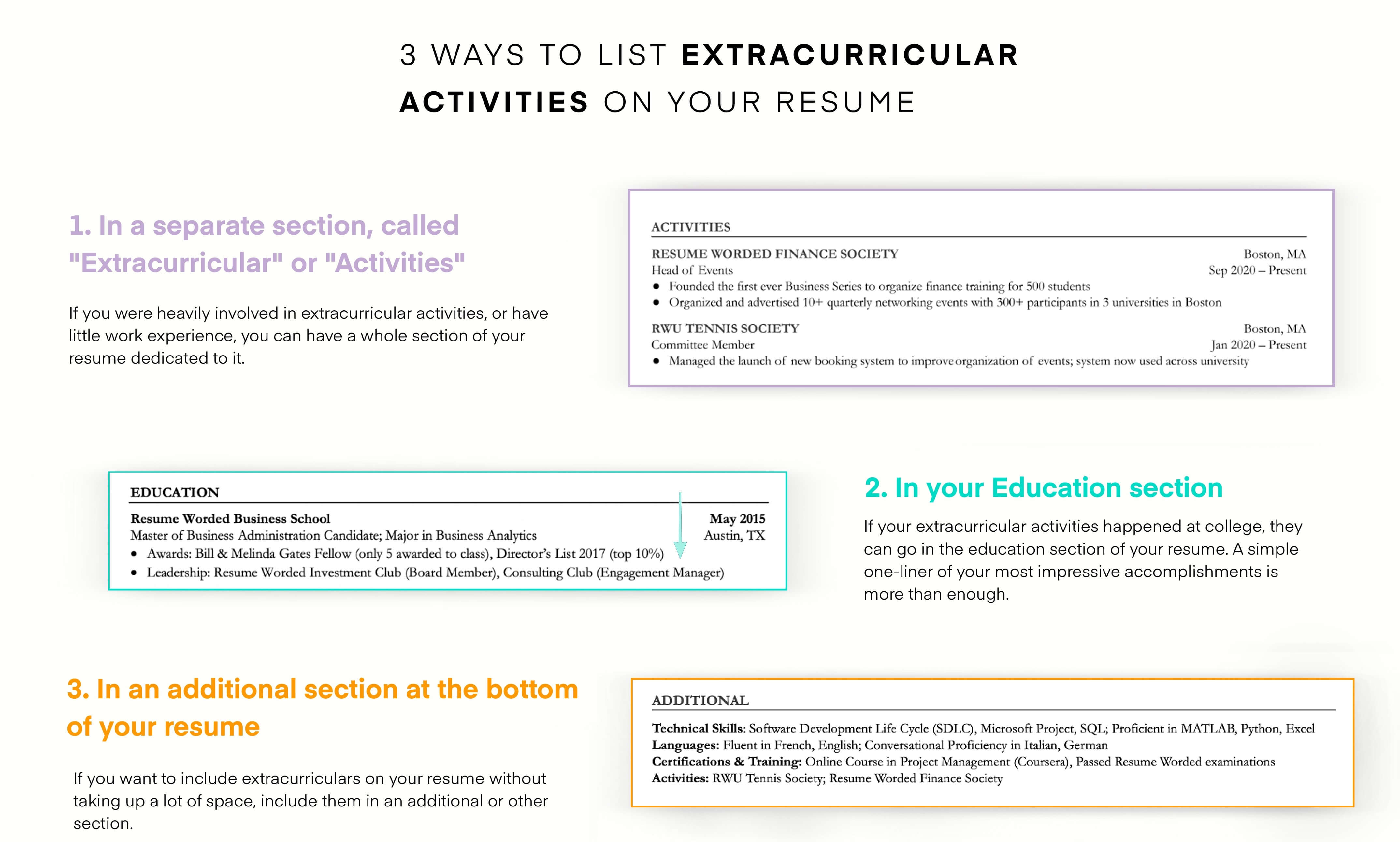
Include extracurricular activities in regulatory affairs.
Considering this is an entry-level position, you may not have extensive work experience. However, this doesn’t mean you can’t demonstrate your value. You can include extracurricular activities in which you practiced regulatory affairs research.