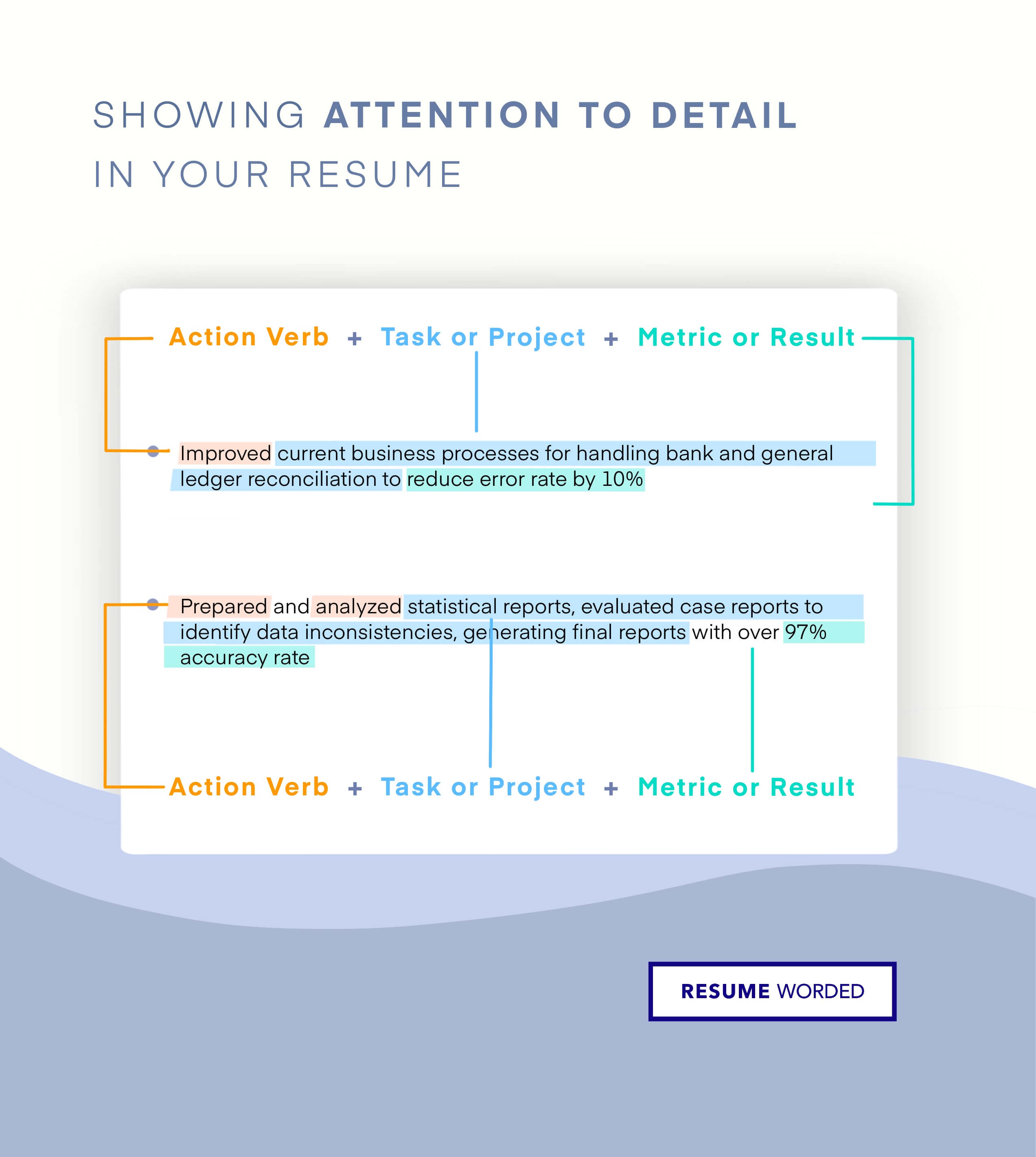
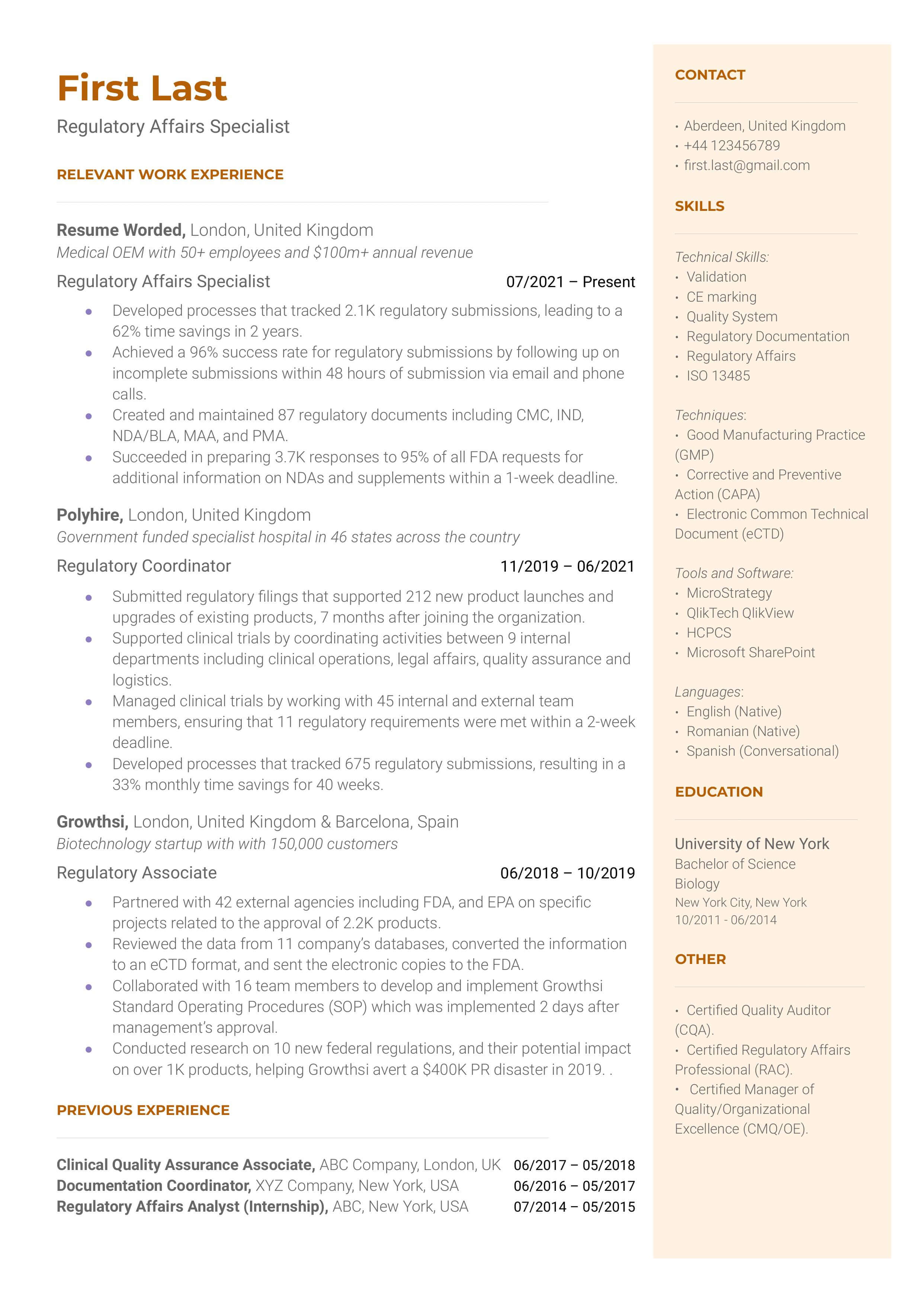
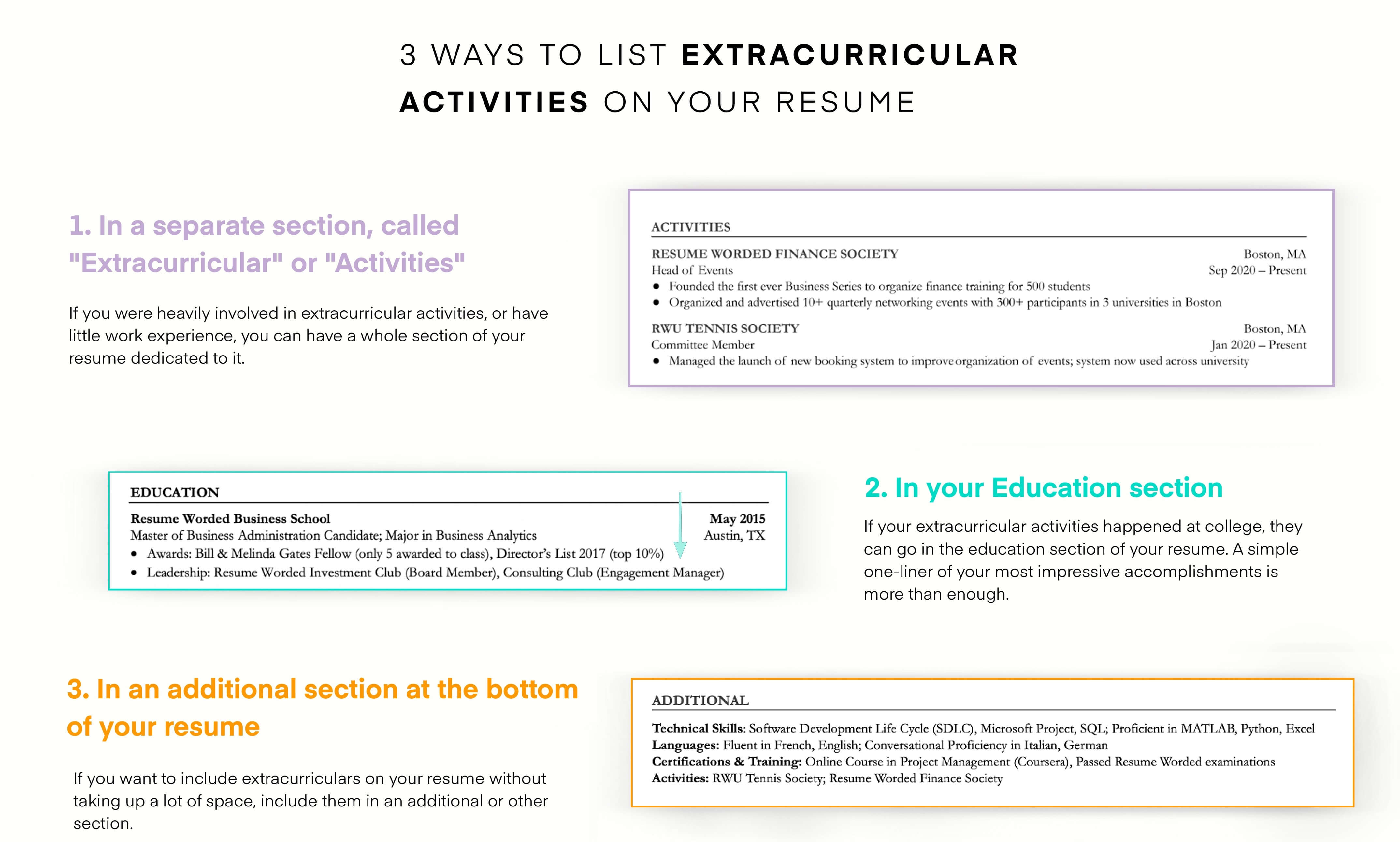
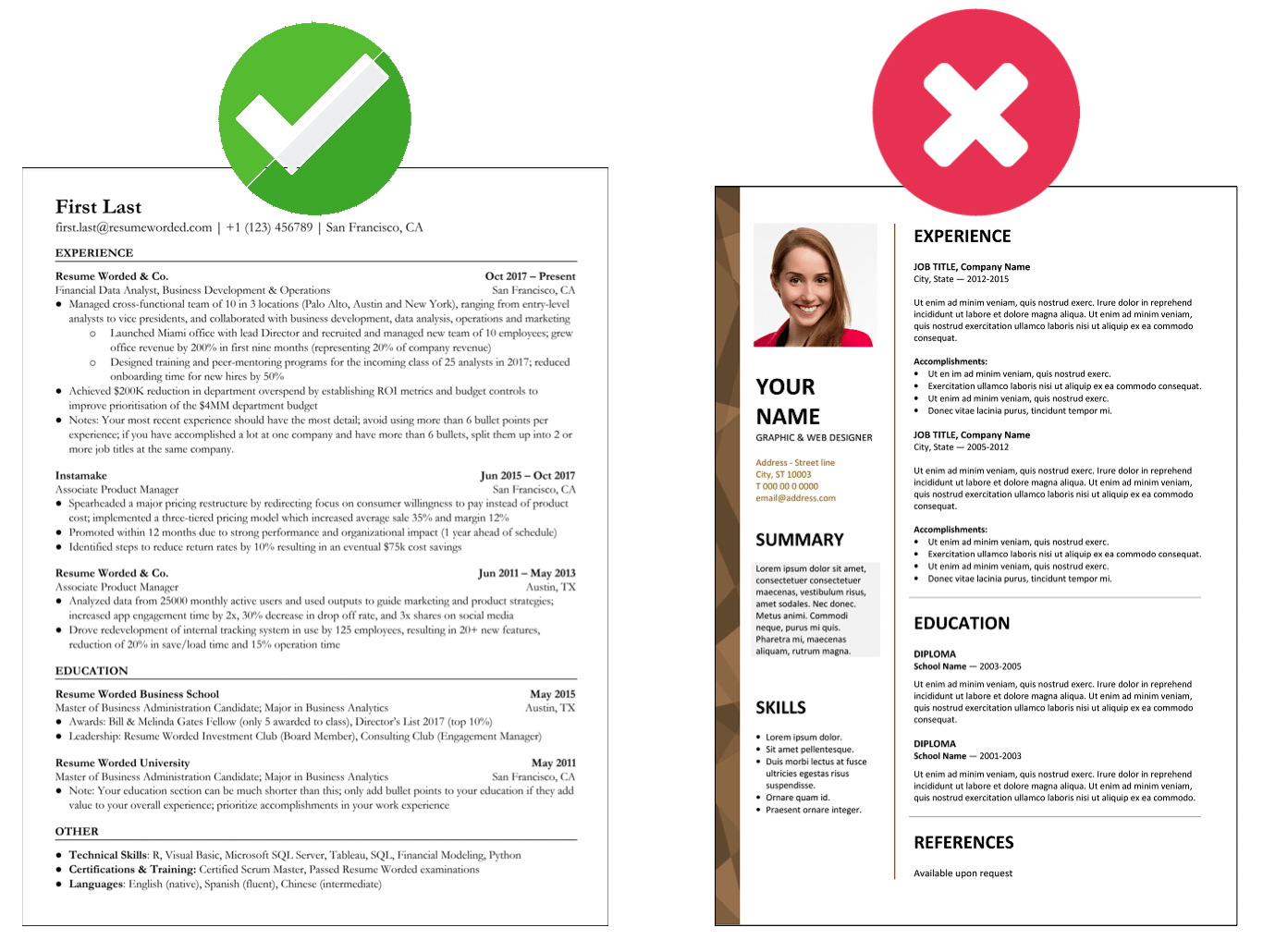
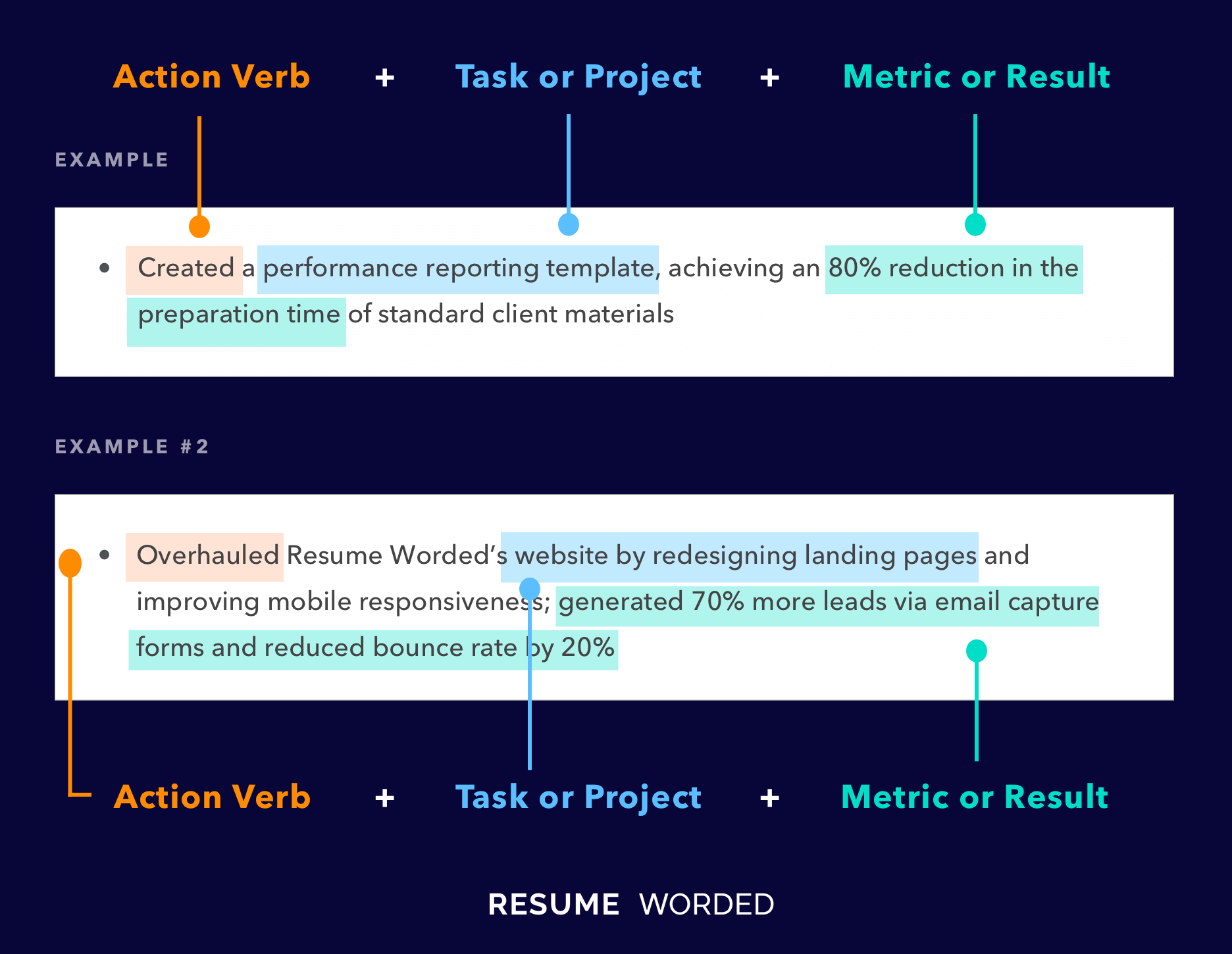
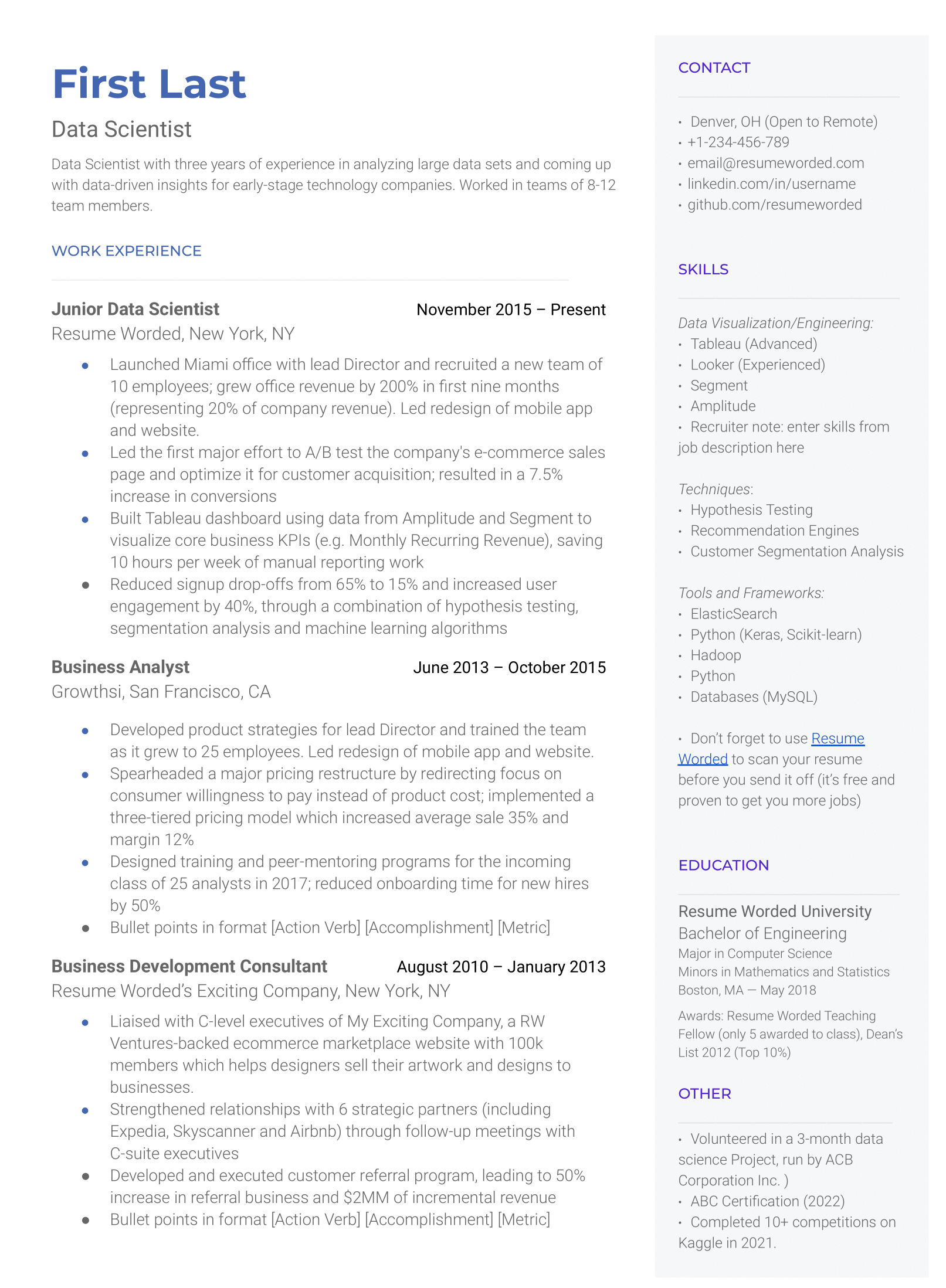
Demonstrate your knowledge of regulatory laws.
As a regulatory specialist, you must be familiar with different laws and regulatory authorities because this is a broad field. You might work in pharmaceuticals, agrochemicals, and even food manufacturing. Each of those fields has different regulatory compliance that you must understand. Try to demonstrate your proficiency in regulatory laws in your resume.