Capitalizing on past success
Mentioning your record of successful drug approval applications, you are essentially providing proof of your ability to manage critical regulatory processes. It offers a glimpse into your competence and significantly increases your employability.
Emphasizing leadership skills
Highlighting your cross-functional team leadership enhances your profile. It shows your ability to collaborate with different departments - a key trait in Regulatory Affairs, where you often have to liaise with various units.
Quantifying achievements
By citing a 40% increase in successful FDA submissions, you're giving concrete evidence of your capabilities. This not only adds credibility to your claims but also gives recruiters a sense of your potential impact on their organization.

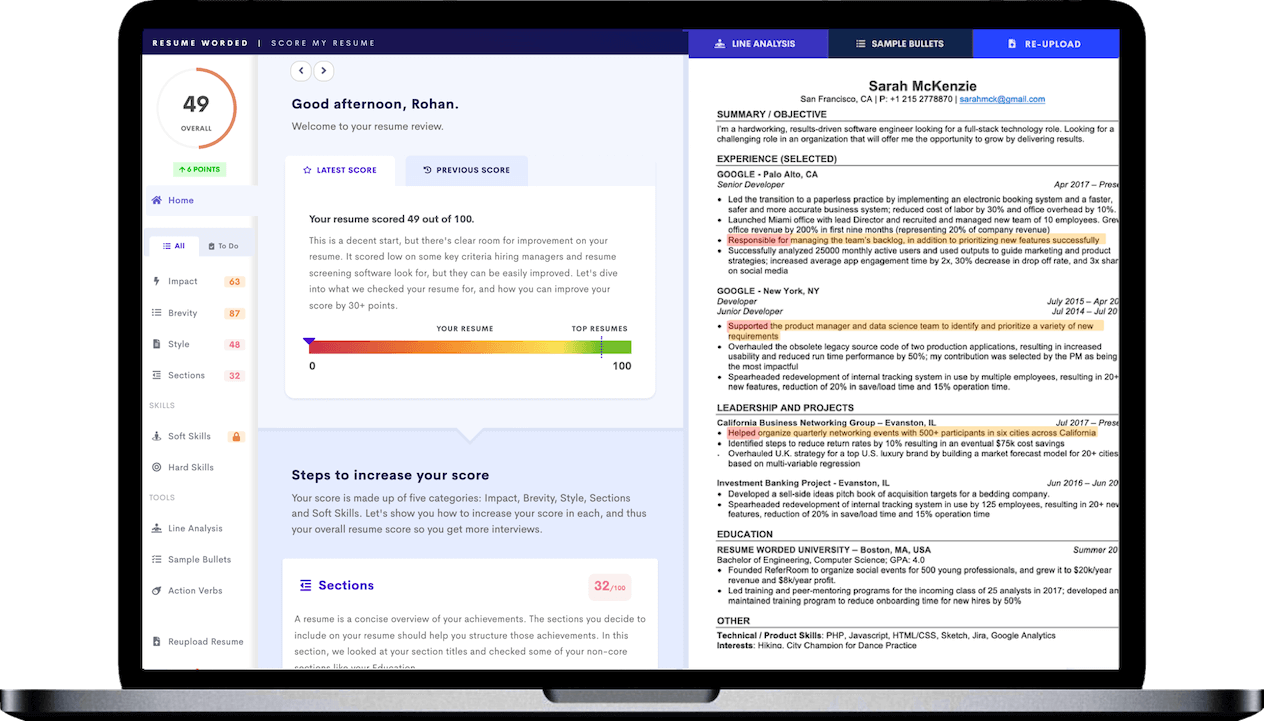







Targeting companies' magnitude
Expressing an interest in working for a multinational organization shows your ambition and readiness to handle complex, global-scale tasks. It also subtly hints at your capacity to navigate diverse work cultures, which is crucial in Regulatory Affairs.