Demonstrates growth through past promotions
This resume template mentions being promoted with a past employer, which is a mark of outstanding performance and professional growth. Both of these factors are crucial for senior-level job candidates.
Here are the keywords and skills that appear most frequently on recent Clinical Operations Specialist job postings. In other words, these are the most sought after skills by recruiters and hiring managers. Go to Sample Templates ↓ below to see how to include them on your resume.
Remember that every job is different. Instead of including all keywords on your resume, identify those that are most relevant to the job you're applying to. Use the free Targeted Resume tool to help with this.
Does your resume contain all the right skills? Paste in your resume in the AI Resume Scan ↓ section below and get an instant score.
Paste your resume below and our AI will identify which keywords are missing from your resume from the list above (and what you need to include). Including the right keywords will help you get past Applicant Tracking Systems (i.e. resume screeners) which may scan your resume for keywords to see if you're a match for the job.
Go through the Clinical Operations Specialist posting you're applying to, and identify hard skills the company is looking for. For example, skills like Clinical Research, Clinical Operations and Clinical Trials are possible skills. These are skills you should try to include on your resume.
The following word cloud highlights the most popular keywords that appear on Clinical Operations Specialist job descriptions. The bigger the word, the more frequently it shows up on employer's job postings. If you have experience with these keywords, include them on your resume.

Here are examples of proven resumes in related jobs and industries, approved by experienced hiring managers. Use them as inspiration when you're writing your own resume. You can even download and edit the resume template in Google Docs.

Business operations managers may have more senior or executive roles at some companies than other operations managers. This resume showcases extensive experience within the field and accomplishments that demonstrate a high level of ability. Past promotions look good on any resume, but they’re especially important for business operations managers because they show leadership and proactivity.
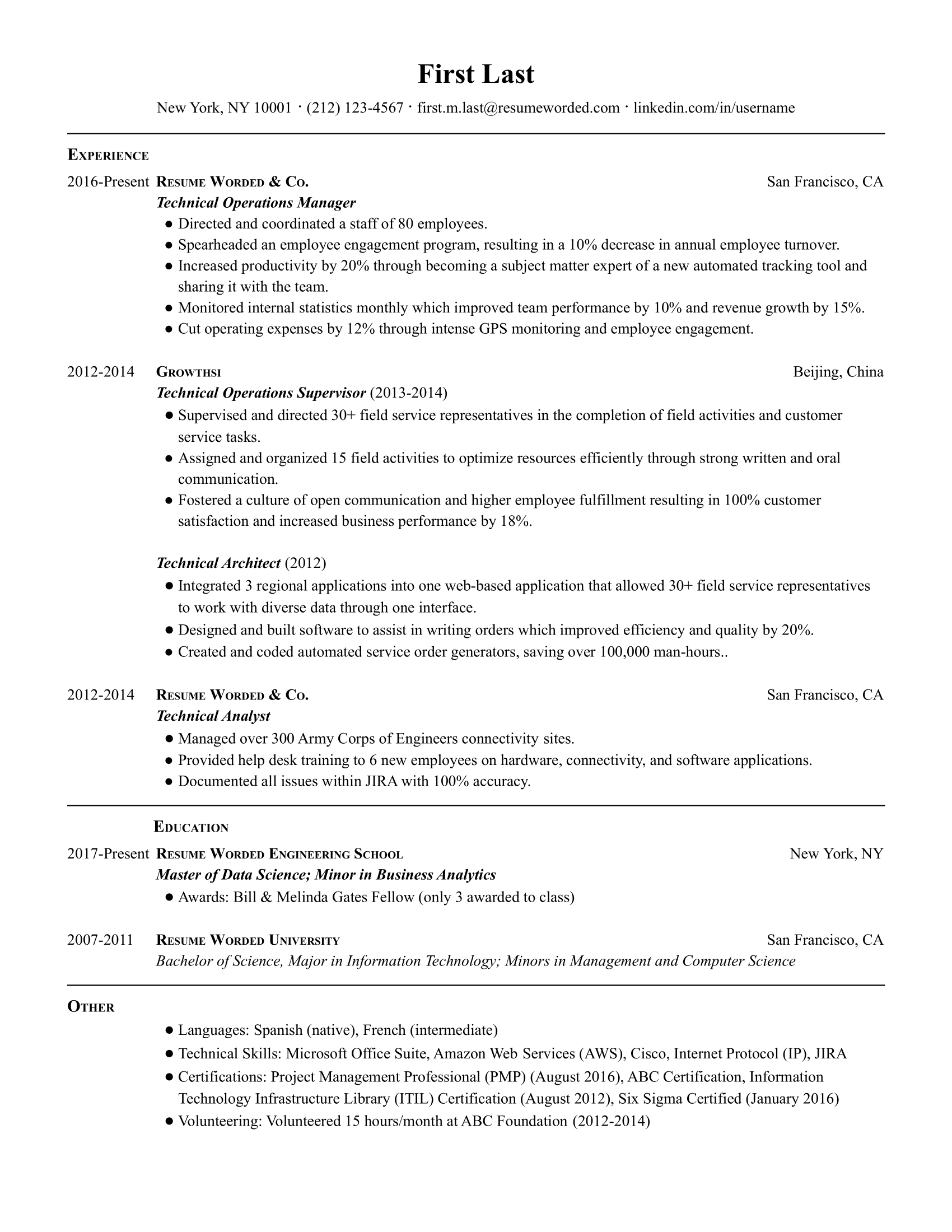
Technical operations managers work closely with technical teams to ensure that projects run smoothly and are completed according to all necessary specifications. They need to balance technical concerns with the interests of the company as a whole. If you’re applying for one of these jobs, use a resume like this one to showcase a blend of technical and managerial abilities.

Some companies employ sales operations managers to oversee their sales teams. These managers have a myriad of responsibilities, including training new sales staff, fostering communication between employees and clients, and streamlining sales processes. When you’re seeking one of these roles, your resume should show that you have some background in sales as well as management.

A marketing operations manager supervises and optimizes companies’ marketing efforts. They may plan and implement campaigns, manage social media accounts, analyze performance, and ensure that all projects and messaging align with the company’s brand. A resume like this one is a strong choice for aspiring marketing operations managers -- it emphasizes skills specific to marketing and work experience in related positions.

Operations associates can learn about business operations as they work to support the day-to-day functioning of a company. While you do need to be organized and conscientious for this type of role, you don’t need management experience for this entry-level role -- so use your resume to underscore your education and internship history, as shown in this resume.
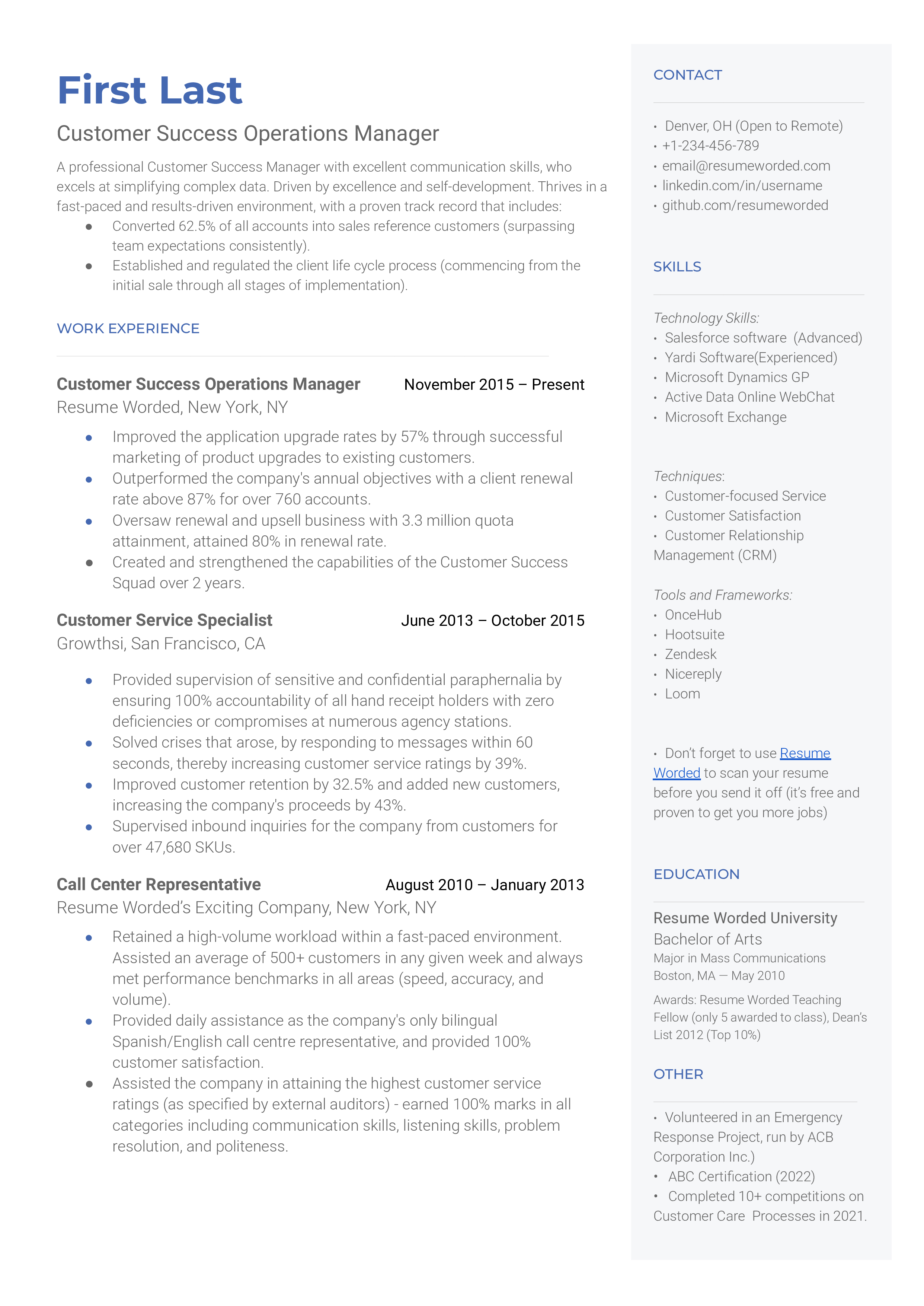
A Customer Success Operations (CS Ops) Manager provides tactical support to the CS team by helping it improve its efficiency and KPIs. To better understand what CS Ops is, think of "success operations" as a product that optimizes processes for Customer Success Managers (CSMs). CS Ops managers establish baseline productivity using various metrics (e.g., net MMR churn) and assess the hindrances to learning about new product features. They evaluate the pain points CSMs face in their daily work and conceptualize how processes work. CSMs identify consistent issues affecting the CS team, break them down into manageable components, and tailor solutions with quantifiable results. In startups and small companies, the CSM mostly doubles as the CS Ops manager. But the need to separate this function into a dedicated role is increasingly apparent. Mostly, the CS Ops manager is a promoted CSM, which means the role calls for extensive experience to immediately build visibility into the CS team's business outcomes, identify areas for improvement, and earmark projects that should be prioritized for Customer Success.
Some effective Clinical Operations Specialist skills you can add to your resume include:
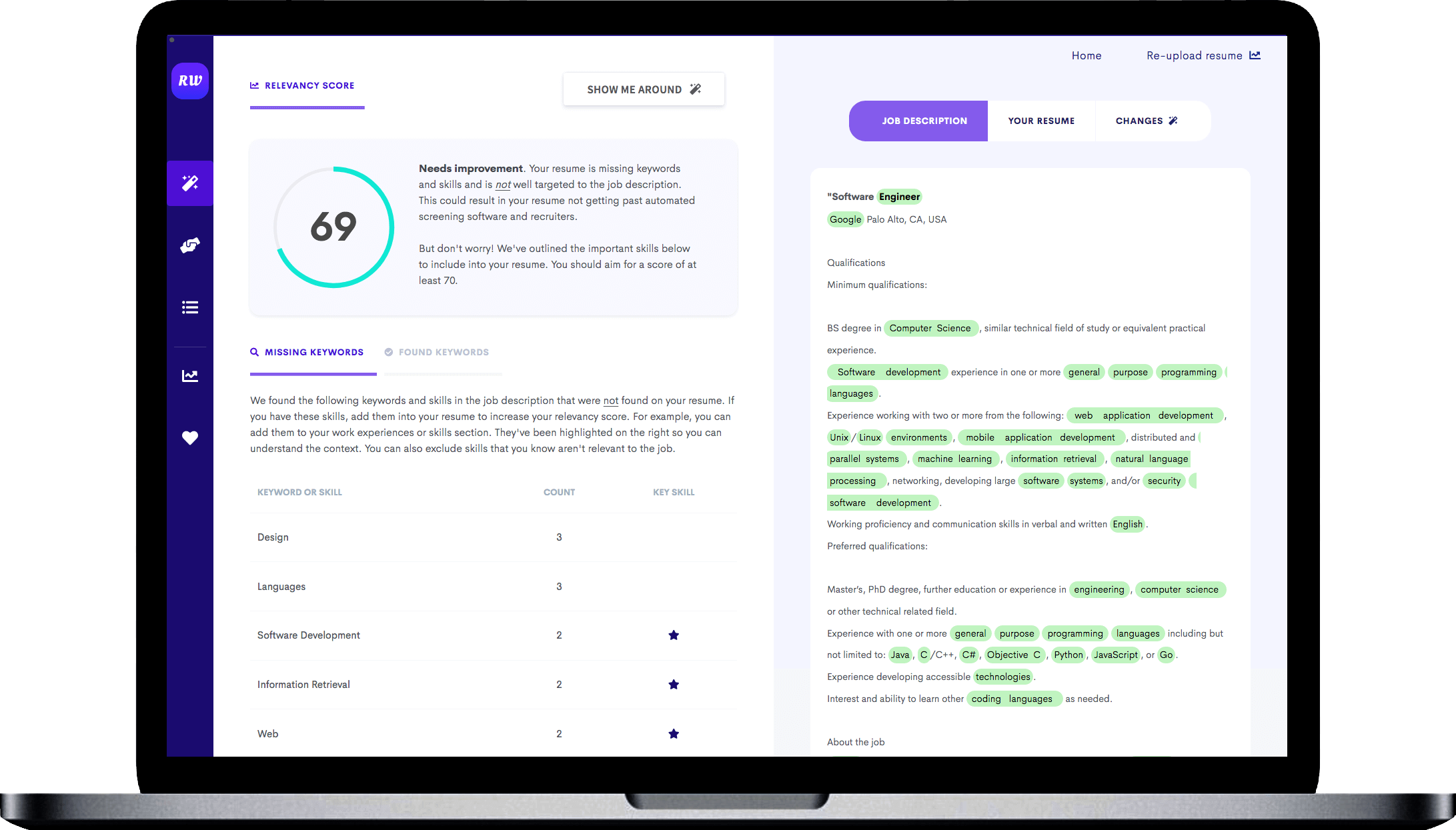
While the keywords above are a good indication of what skills you need on your resume, you should try to find additional keywords that are specific to the job. To do this, use the free Targeted Resume tool. It analyzes the job you are applying to and finds the most important keywords you need on your resume.
It is personalized to your resume, and is the best way to ensure your resume will pass the automated resume filters.

"My free resume review was truly eye-opening. I found out why I wasn't getting interviews and exactly what to add to get past resume screeners. I've already had way more callbacks since I used it. I recommend it to all my friends who are job searching."

"Probably the best thing I've done this year. Showed me what my strengths were and the jobs and industries I should be focusing on. The most impactful part though was how it identified this spiral I'd been doing subconsciously - yikes, freakishly accurate."

Thank you for the checklist! I realized I was making so many mistakes on my resume that I've now fixed. I'm much more confident in my resume now.