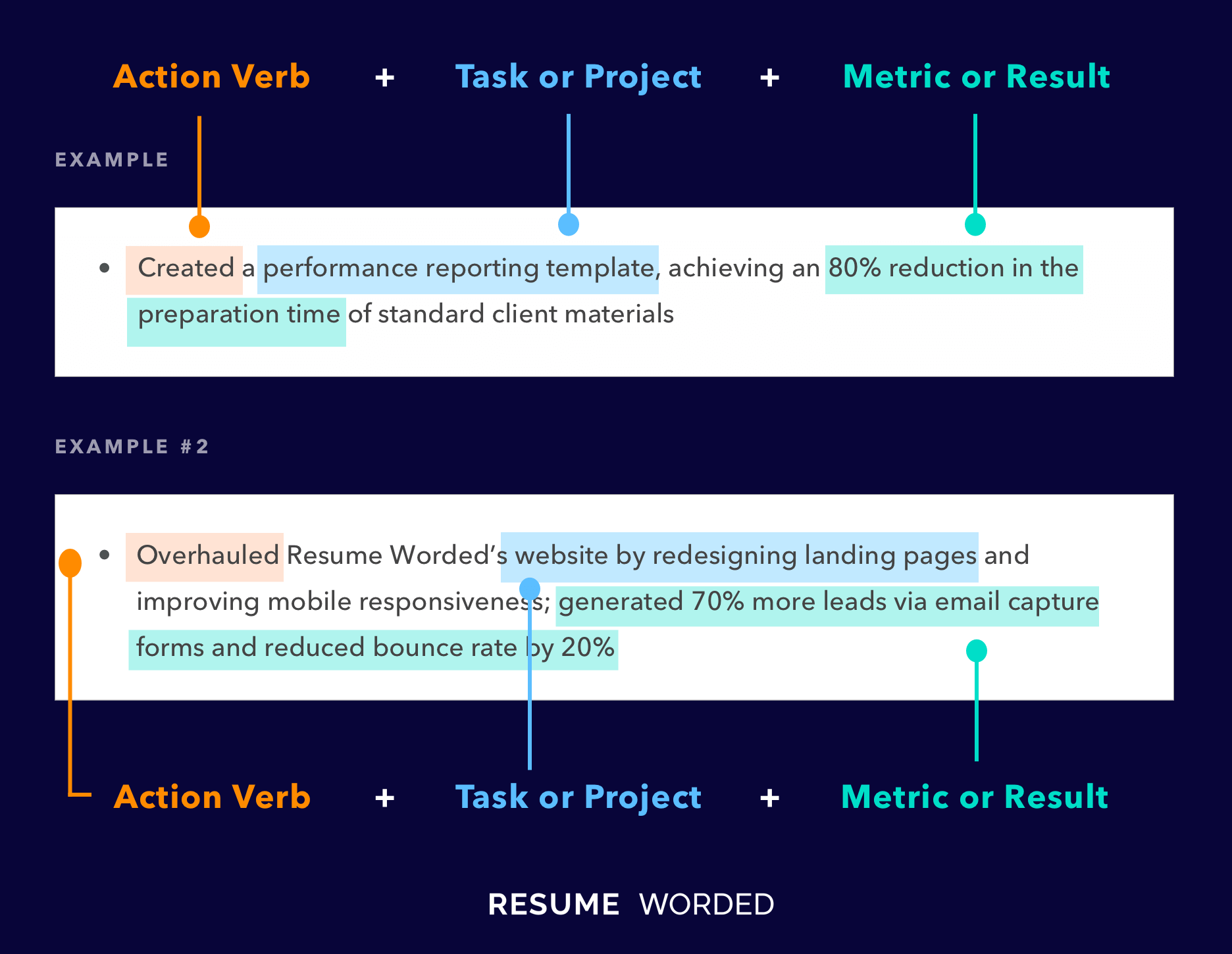
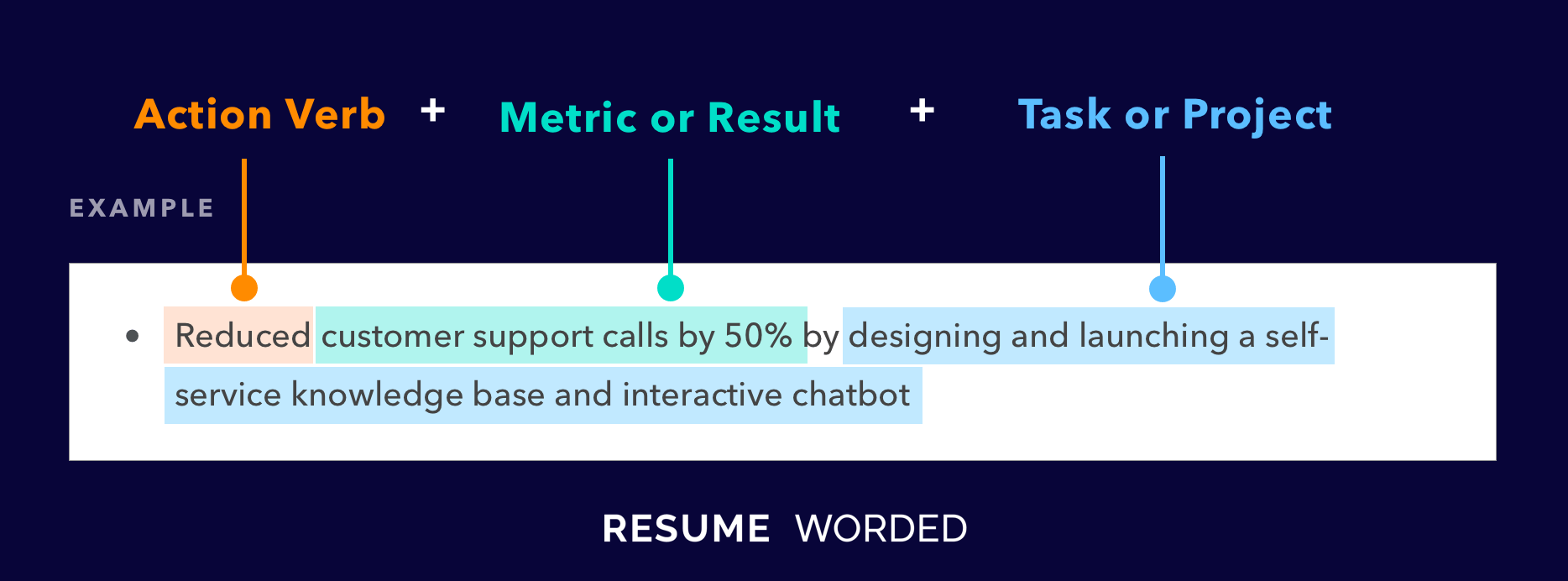
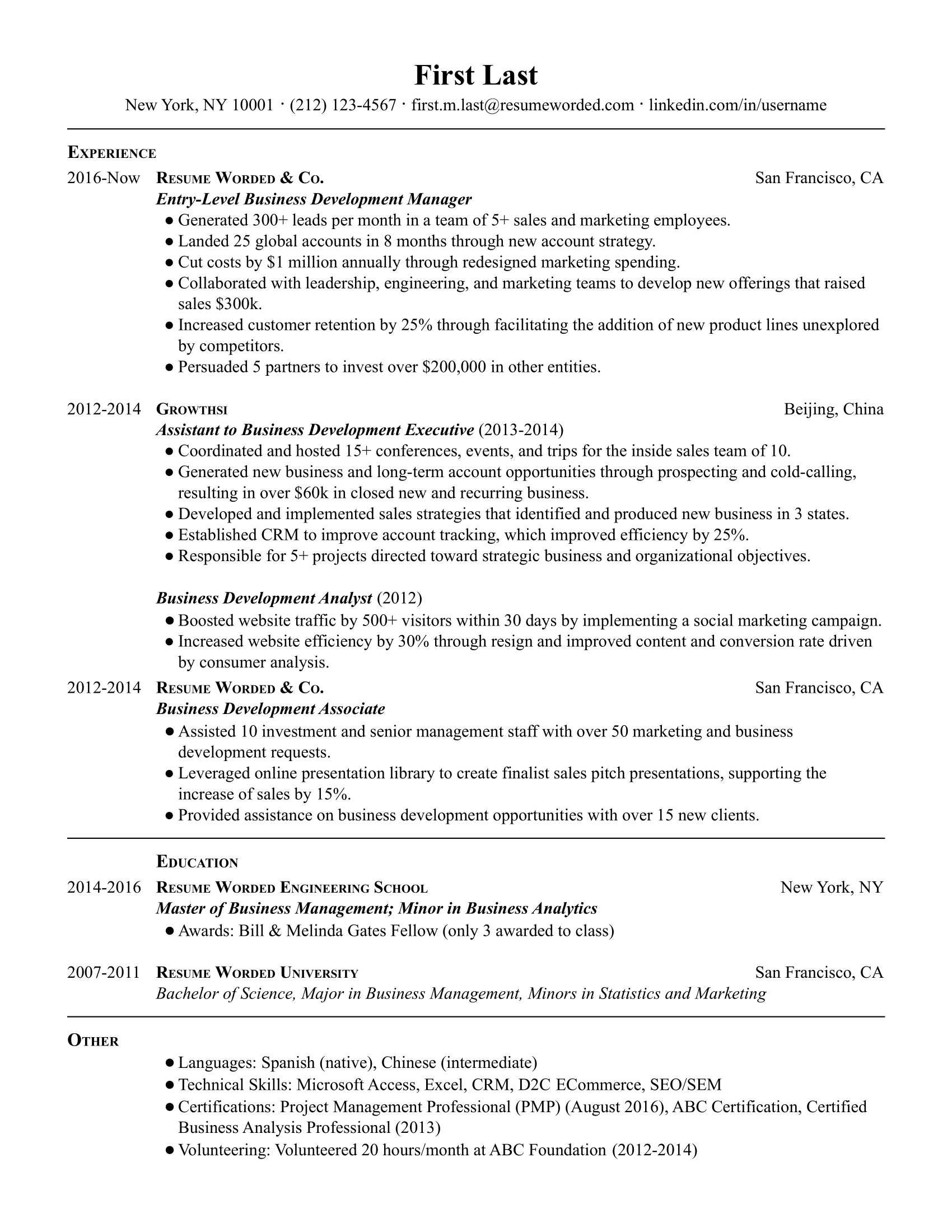
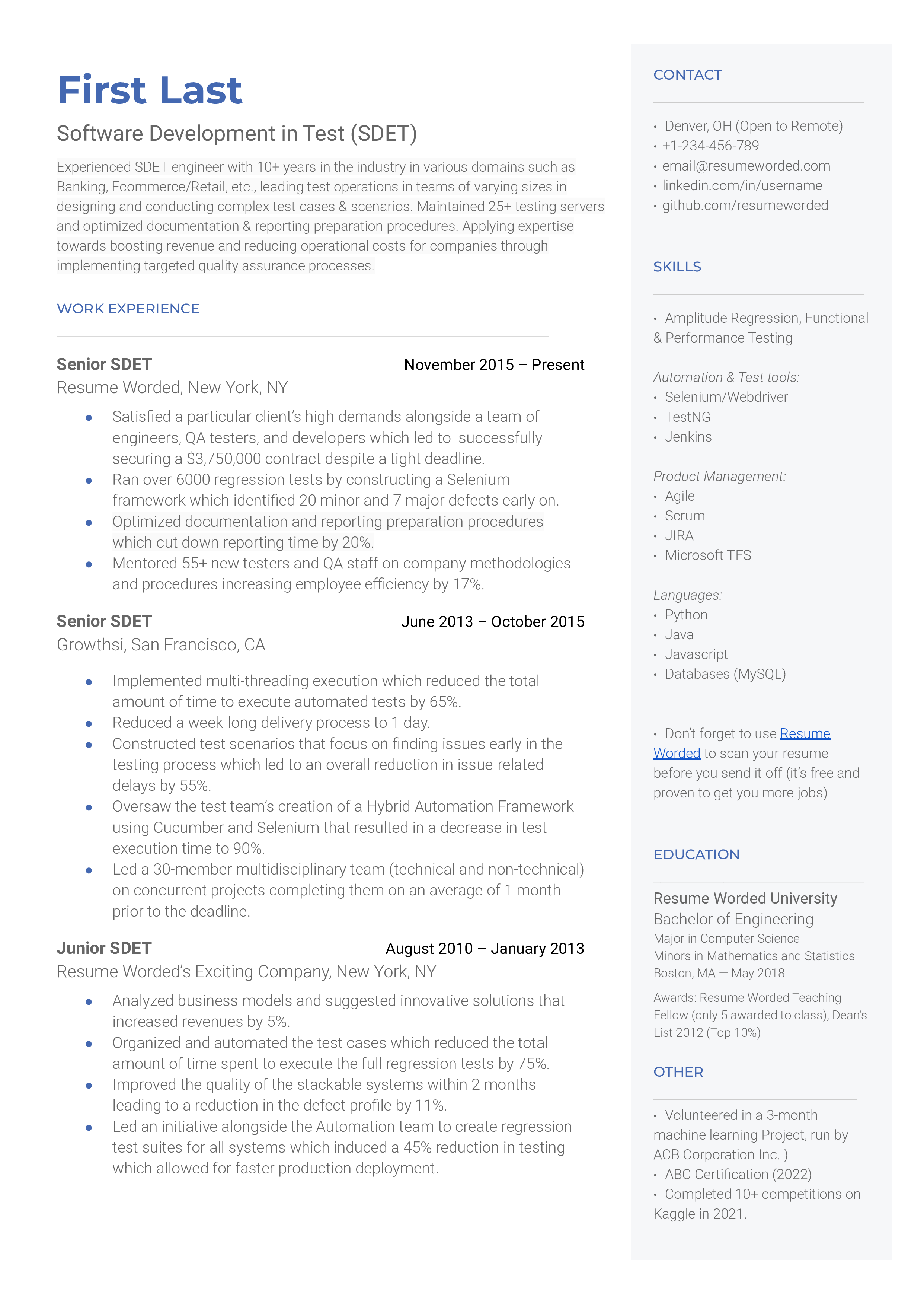
Well-rounded and related accomplishments to business development specialists
Rather than repeating similar accomplishments across their positions, this applicant has included a variety of accomplishments that all fall under the business development umbrella. For example, they reference their ability to generate leads through cold calling, their skills at managing customer relationships, and assisting in training representatives. If you have a similar breadth of skills, you should do the same to indicate your overall capabilities in the position.