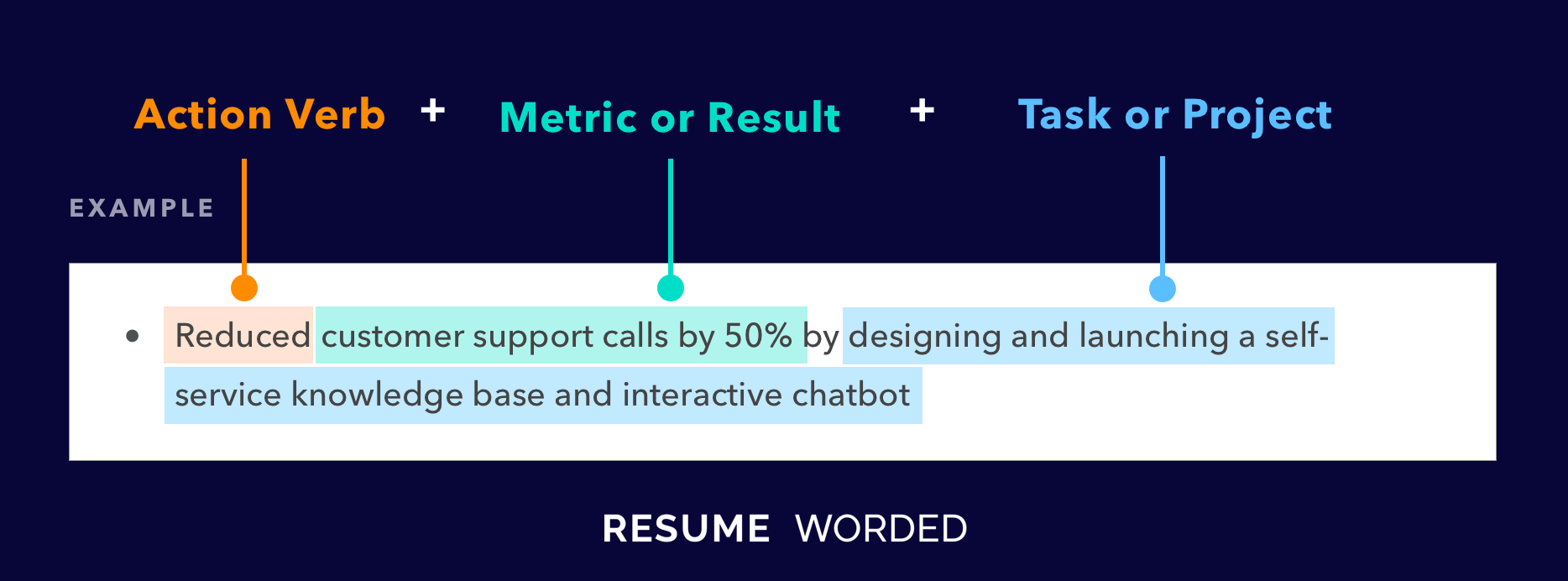
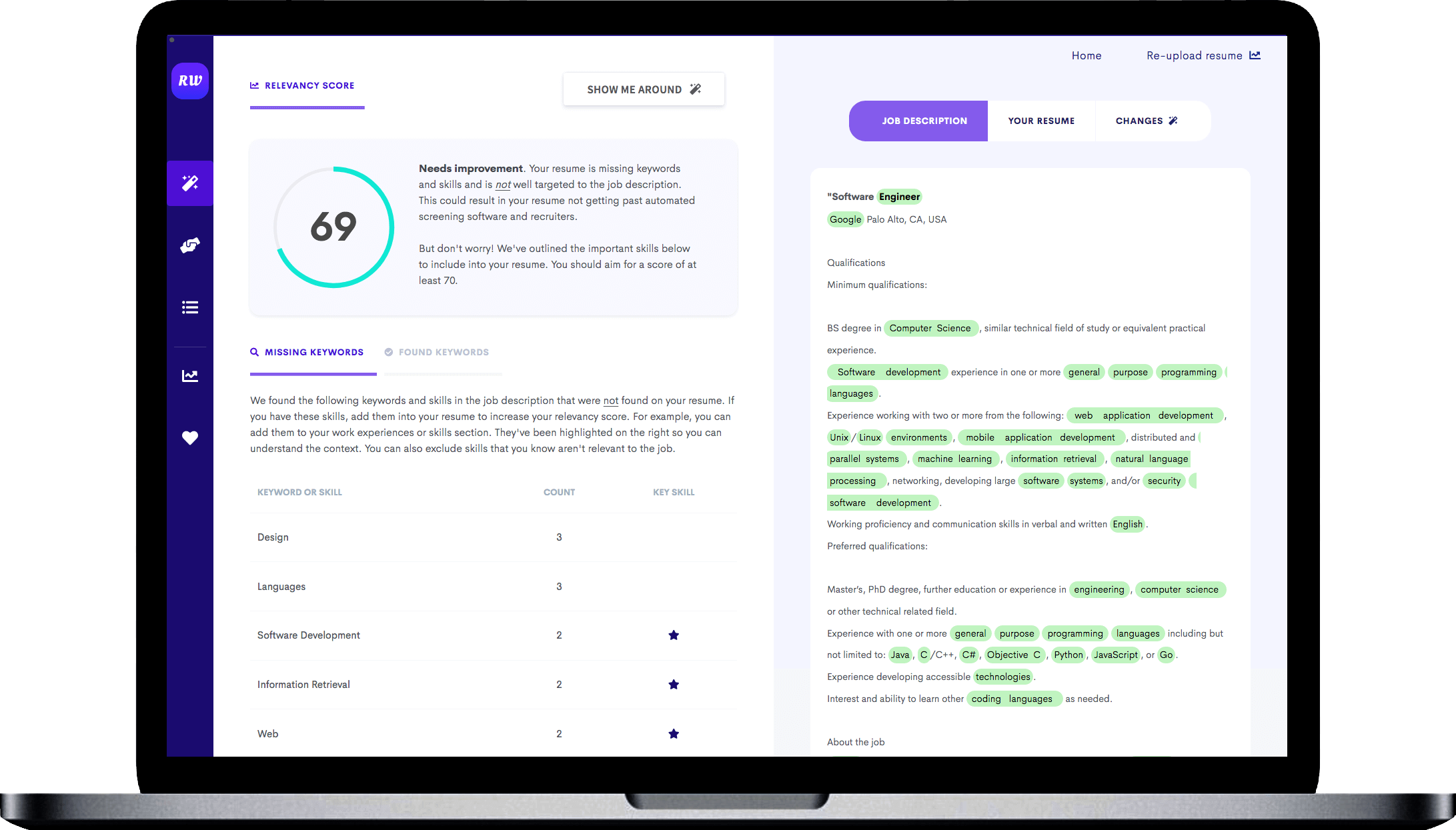
Emphasis on managerial skills
You can see in the experience section of this sample how they led a few projects. They discuss what was done, who they worked with, and how big a team they had. Follow a similar layout in your resume so recruiters can see that you can lead data science teams.