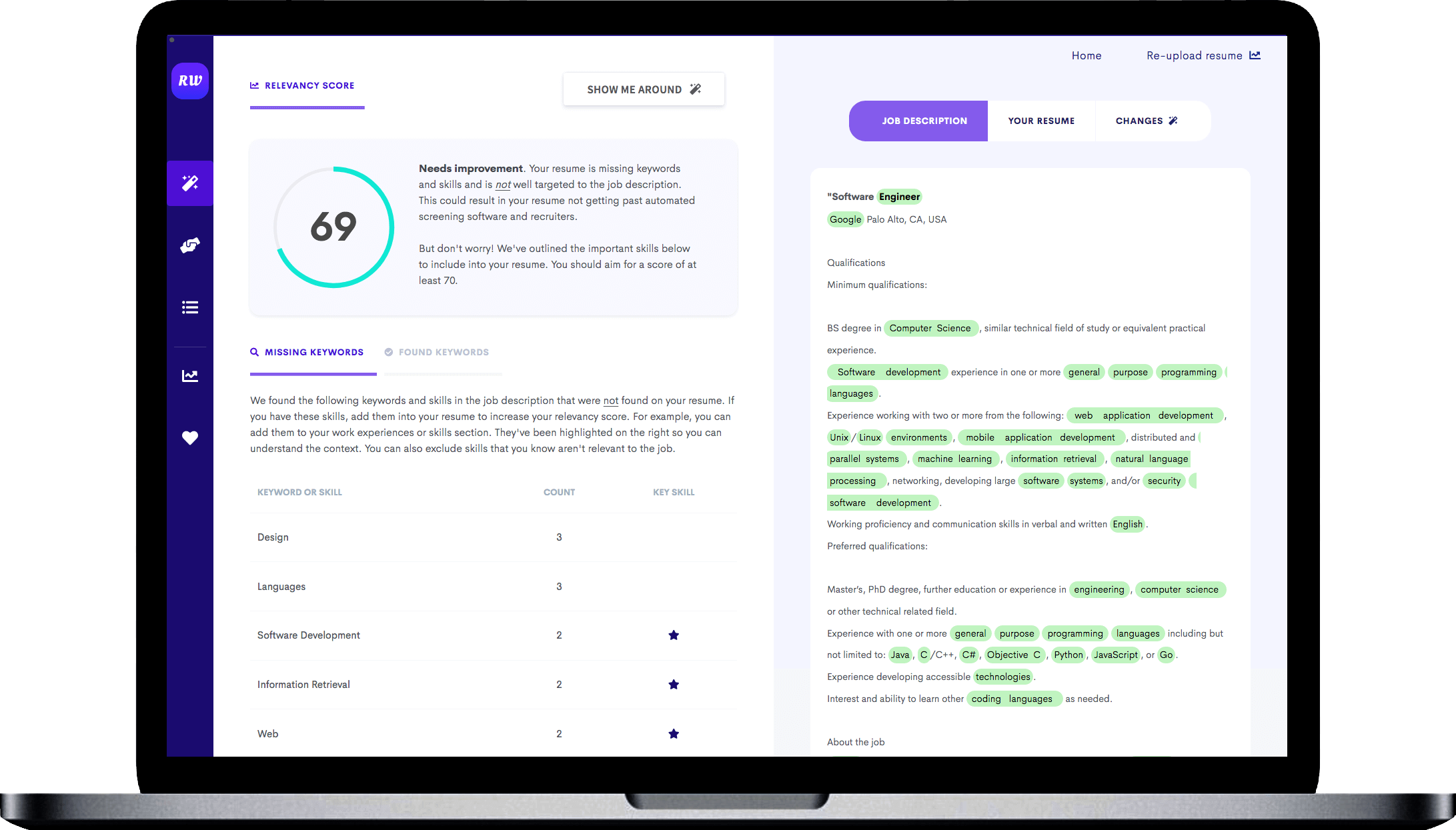
Highlight relevant projects that relate to the role of a medical coder
Being that this is an entry level job, hiring managers will be looking to see if you were engaging in concepts relevant to the position throughout your undergraduate career. A great way to showcase your knowledge in the field is to detail research papers or projects you’ve completed in college. Any project you’ve completed in information technology is valuable, but particularly if the subject matter was specific to medical coding.