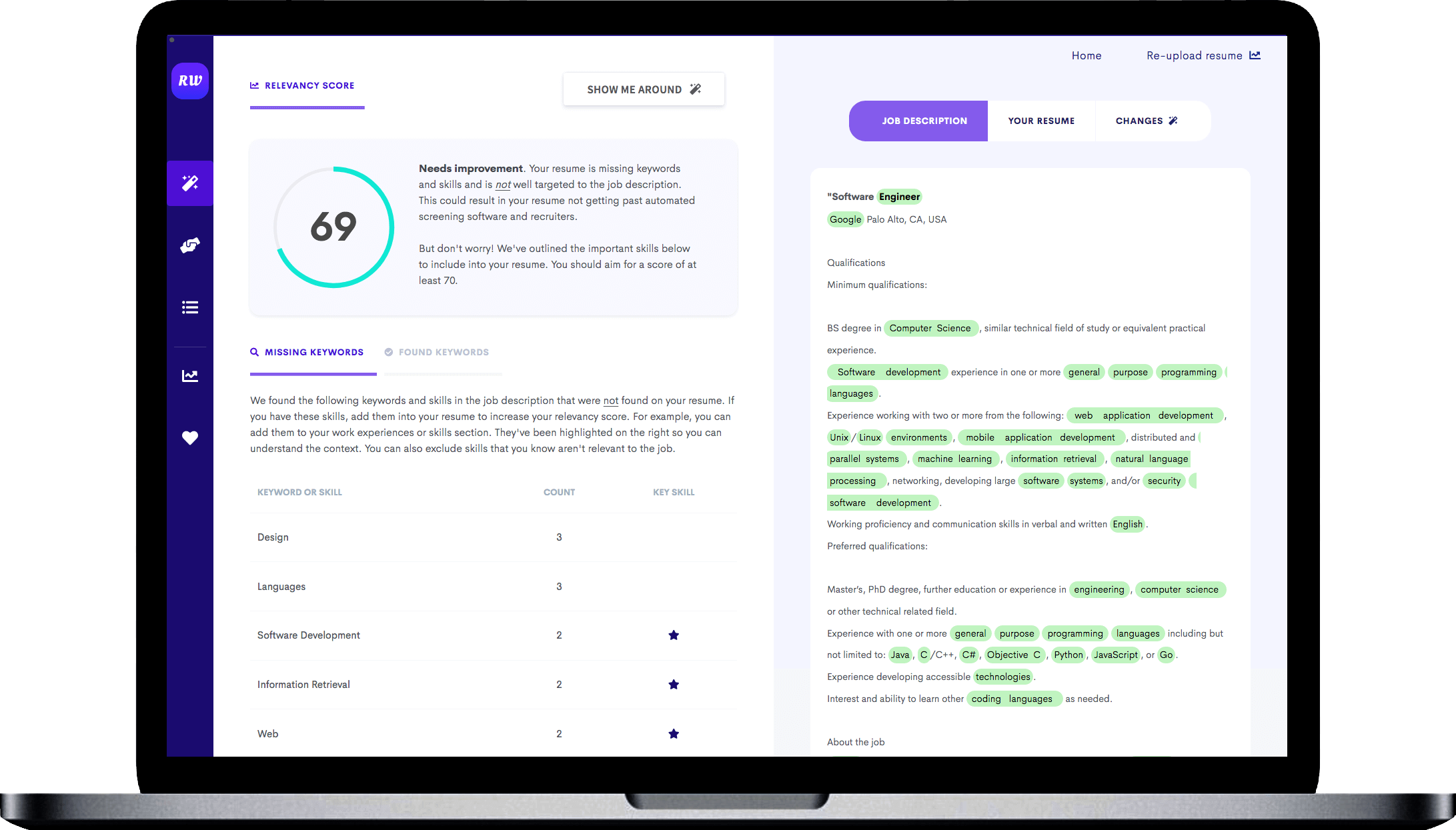
Indicate your familiarity with Enterprise Resource Management (ERM) systems.
Procurement coordinators must keep track of purchases and inventory management, so it is vital to understand how ERM systems work. If you are familiar with this program, you should mention it in your resume. An ERM system is an inventory management software that gathers an organization’s business data. With this system, you can monitor goods purchases, regulate inventory requests, and invoice receipts.