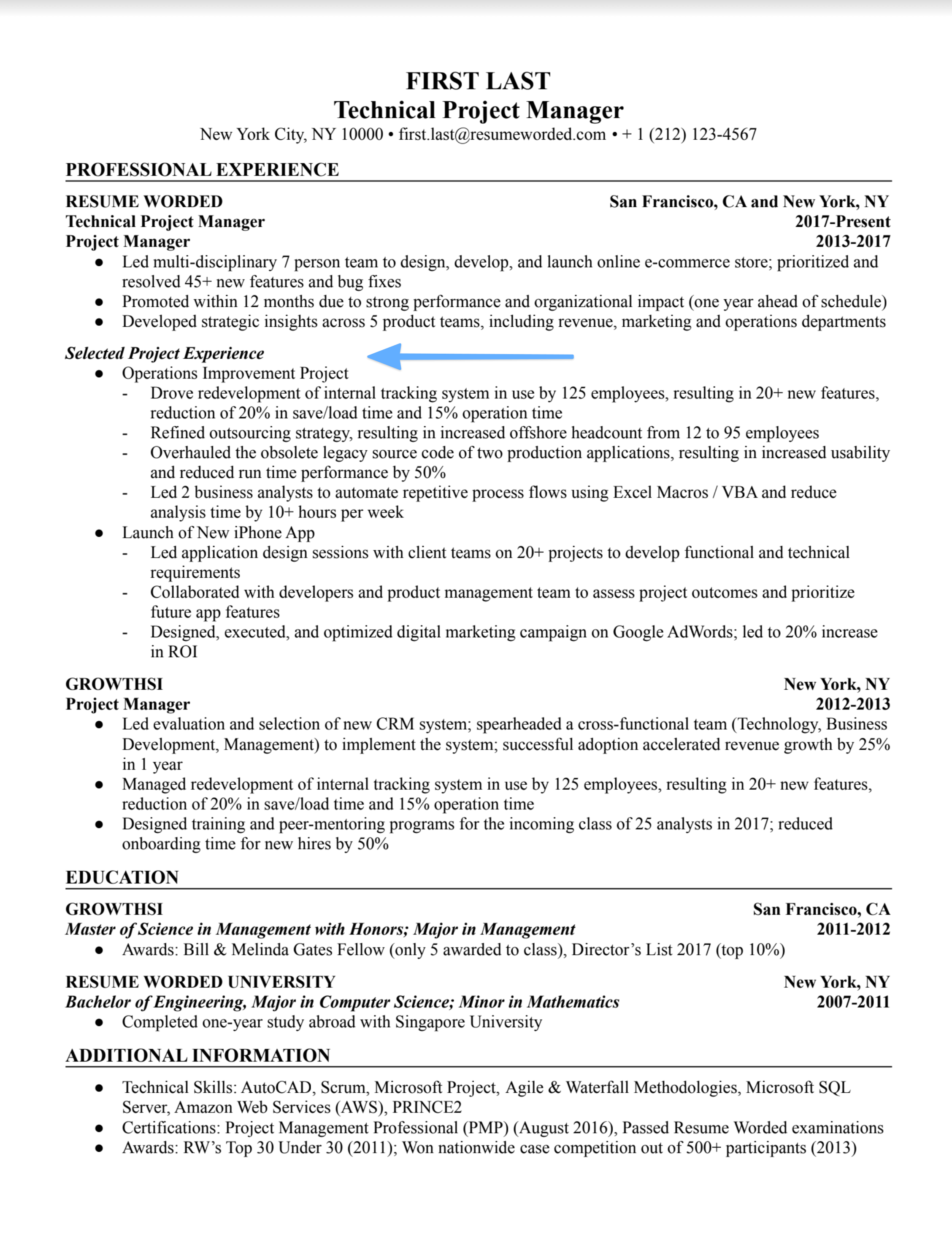
Education and academic background
On an entry-level resume, leading with your educational history can put your strengths in the forefront - especially if you excelled in school. If you had a high GPA or academic honors, it’s great to mention those here, as well as any coursework or projects you did that could be relevant for project management.