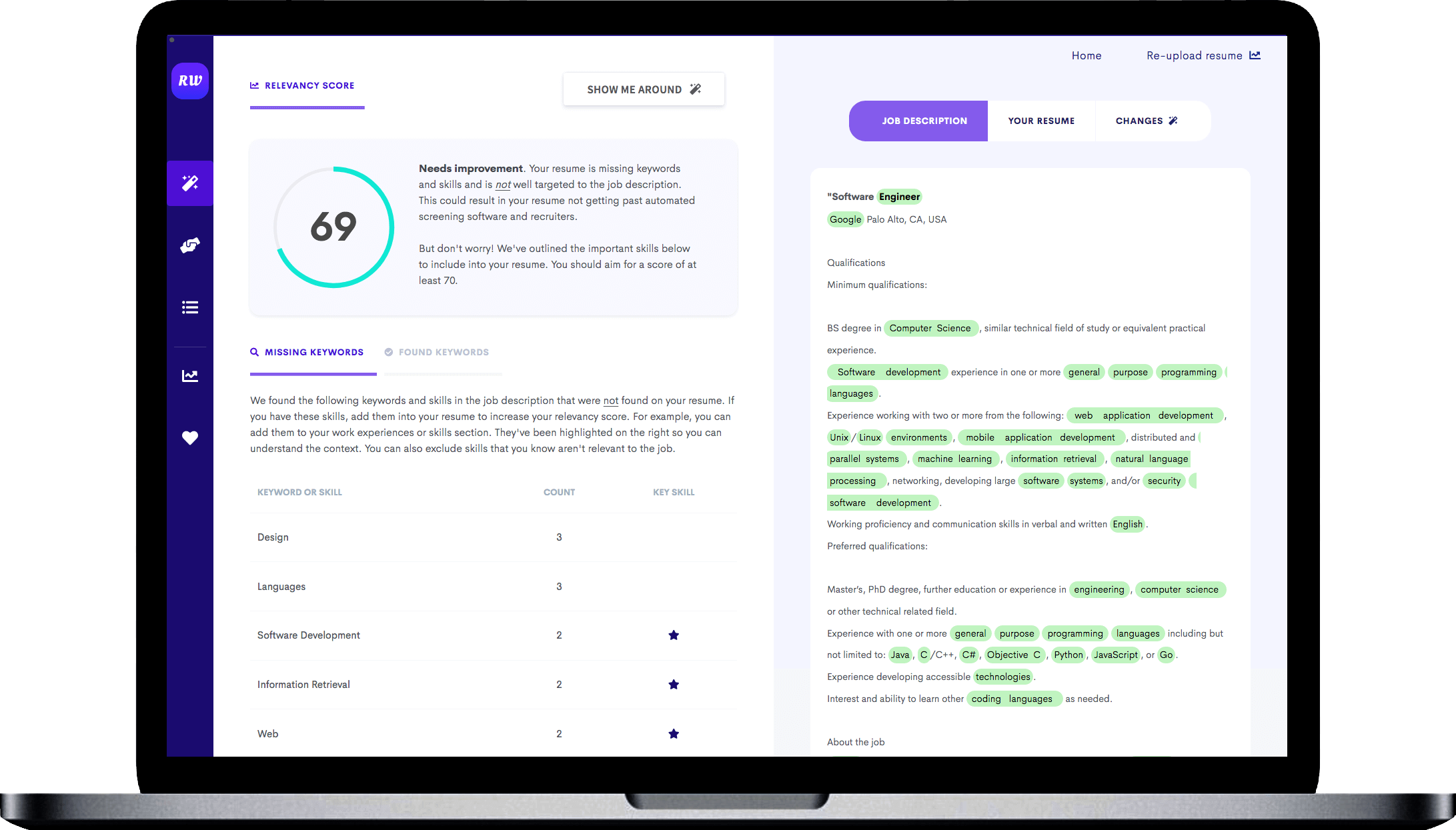
Include certifications and relevant qualifications
Beyond your educational background, it's crucial to include any certifications or qualifications related to clinical trial management. Certifications like Certified Clinical Research Professional (CCRP) or Certified Clinical Research Associate (CCRA) show you're well-qualified and committed to staying current in the field.